Briefly Explain the Difference Between Oxidation and Reduction
Therefore oxidizing agents oxidizes other substances and reducing agents reduces them. 3 on a question 161 a briefly explain the difference between oxidation and reduction electrochemical reactions.
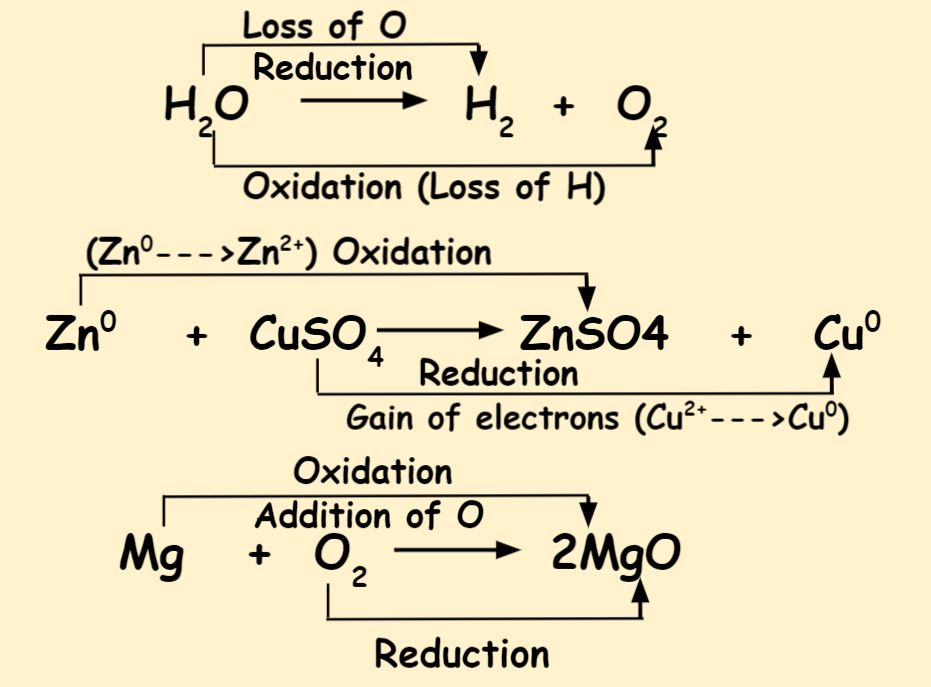
10 Differences Between Oxidation And Reduction Reaction Dewwool
1 HCl 2 an HCl solution containing.

. 18 b Which reaction occurs at the anode and which at the cathode. Loss of electrons Eg. 18 b Which reaction occurs at the anode and which at the cathode.
Reduction is the process by which an atom an extra electron for electrons and becomes an anion bOxidation occurs at the reduction at the Q Mu. Continue to order Get a quote. 172 a Write the possible oxidation and reduction half-reactions that occur when magnesium is immersed in each of the following solutions.
Loss of hydrogen 2. B which reaction occurs at the anode and which at the cathode. A Briefly explain the difference between oxidation and.
In oxidation reactions electrons are lost but in reduction reactions electrons are gained. The main difference between oxidation and reduction is that oxidation is the increasing of oxidation state of an atom whereas reduction is the decreasing. Briefly explain the difference between oxidation and reduction electrochemical reaction and state which reaction occurs at the anode and which.
Loss of oxygen 2. Answer a Oxidation is the process by which an atom gives up an electron or electrons to become a cation. Passed from one material to the other.
B Which reaction occurs at the anode and which at the cathode. During a reaction oxidizing agent undergoes reduction. A redox reaction is a chemical reaction that occurs through the electron exchange between atoms.
In oxidation hydrogen is lost but in reduction hydrogen is gained. In oxidation reactions oxygens are gained and in the reduction reactions oxygens are lost. Vit Answeraoxidation is the process by which an atom an electron for electrons to become a cation.
Oxidation is the loss of electrons from atoms molecules or ions. 181 a Briefly explain the difference between oxidation and reduction electrochemical reactions. Gain of oxygen 1.
171 a Briefly explain the difference between oxidation and reduction electrochemical reactions. Oxidation involves an increase in oxidation number while reduction involves a decrease in oxidation number. 2 1 C l 2 e C l.
Both oxidation and reductions typically occur at the same time. Decrease in oxidation number. A Oxidation is the loss of an electron while reduction is the gaining of an electron b Reduction is at the cathode while oxidation is at the anode.
The electron is typically. Oxidation is the process by which an atom gives up an electron to become a cation. Reduction and oxidation both occur simultaneously.
Gain of electrons Eg. Reduction occurs at the cathode. What is the difference between Oxidation Reaction and Reduction Reaction.
B Oxidation occurs at the anode. Reduction is the process by which an atom acquires an extra electron or electrons and becomes an anion. This is because in reduction a material is gaining an electron while in oxidation the material is loosing the electron.
Oxidation reactions always occur with a reduction reaction simultaneously. Depending on the chemical reaction oxidation. B Which reaction occurs at the anode and which at the cathode.
Gain of hydrogen 3. Briefly explain the difference between oxidation and reduction electrochemical reaction also state which reaction occurs at anode abs which at cathode. Oxidizing agents removes electrons from another substance in a redox reaction whereas reducing agents donates electrons.
Increase in oxidation number 4. The main difference between the reduction and oxidation process is based on gaining and loosing of electron. One is loosing electron and other is gaining.
In contrast the reducing agent undergoes oxidation. The electron is typically passed from one material to the other. The one that is loosing electron is oxidation process and the one that is gaining electron is reduction.
Oxidation the material is losing the electron. Oxidation and reduction are the two half reactions of redox reactions. Usually the change in oxidation number is associated with a gain or loss of electrons but there are some redox reactions eg covalent bonding that do not involve electron transfer.
Oxidation and Reduction. A Briefly explain the difference between oxidation and reduction electrochemical reactions. M g M g 2 2 e 3.
Both oxidation and reductions typically occur at the same time. The main difference between corrosion and oxidation is that corrosion happens chiefly on metal surfaces whereas oxidation can happen anywhere. Both oxidation and reductions typically occur.
5 rows Difference between Oxidation and Reduction. Main Difference Oxidation vs Reduction. Reduction is the process by which an atom acquires an extra electron and becomes an anion.
181 a Briefly explain the difference between oxidation and reduction electrochemical reactions.

10 Differences Between Oxidation And Reduction Reaction Dewwool
Difference Between Oxidation And Reduction Definition Mechanism Examples

Difference Between Oxidation And Reduction Reaction

Difference Between Oxidation And Reduction Compare The Difference Between Similar Terms
0 Response to "Briefly Explain the Difference Between Oxidation and Reduction"
Post a Comment